Pfizer (PFE) Starts EU Submission of Variant-Adapted Vaccine
Pfizer PFE and its partner BioNTech BNTX announced that the European Medicines Agency (EMA) initiated the rolling review of their regulatory application for a variant-adapted version of the companies’ COVID-19 vaccine.
Pfizer and BioNTech stated that the EMA has started evaluating the chemistry, manufacturing, and controls (CMC) data shared by them with the regulatory body. The companies will submit clinical data, including data on immunogenicity against Omicron and its subvariants for their rolling submission as and when it becomes available.
Pfizer and BioNTech are also planning to file data supporting a potential variant-adapted vaccine to the FDA in the coming weeks.
The companies are developing several variant-adapted vaccines. They are planning to discuss the same with global regulatory bodies to determine the composition of the variant-adapted vaccine for a potential vaccine booster approach for the Fall 2022 season.
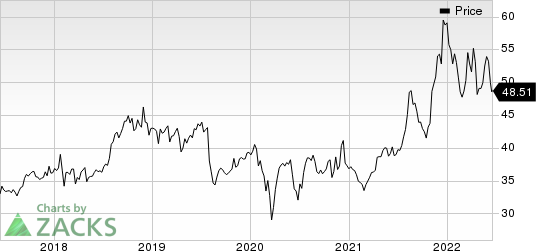
Pfizer’s stock is down 17.8% this year so far against an increase of 2.7% for the industry.

Image Source: Zacks Investment Research
Pfizer and BioNTech received a recommendation from an FDA advisory committee for the use of their COVID-19 vaccine in infants six months to five years of age. Kids under five years of age are not yet authorized to get any COVID-19 vaccine in the United States, even as a primary regimen.
Pfizer and BioNTech are seeking approval for their mRNA-based COVID-19 vaccine for children six months to under five years of age as a three-dose vaccine. The vaccine elicited a strong immune response, high efficacy and a favorable safety profile when given as a three-dose vaccine to children six months to under five years of age in clinical studies
Top-line data from a phase II/III study showed that the three-dose vaccine was as effective in the 6- to 24-month-old population and the 2- to under 5-year-old population as the second dose in the 16- to 25-year-old population. The vaccine’s efficacy was 80.3% in the above-mentioned age group. This vaccine efficacy, a secondary endpoint in the study, was observed in the descriptive analysis of three doses while the Omicron variant remained dominant.
The FDA reviewers said that the available data supports Pfizer and BioNTech’s vaccine’s effectiveness in preventing COVID infection in this age group as a three-dose primary series.
Pfizer’s formulation of a booster dose for these youngest children is 3-µg, which is one-tenth of the dose strength for adults.
Rival Moderna’s MRNA mRNA-based vaccine is also not yet authorized for use in kids under 6 years of age. Moderna has also filed data with the FDA, seeking emergency approval for two 25-µg doses of its vaccine in children six months to under six years of age. Its two-dose vaccine was 37%-51% effective in this age group in an analysis when the Omicron variant was circulating. Last week, similar briefing documents for Moderna’s vaccine were released, which said that the vaccine works safely and effectively in children six months to under six years of age.
Pfizer Inc. Price
Pfizer Inc. price | Pfizer Inc. Quote
Zacks Rank & Stock to Consider
Pfizer currently carries a Zacks Rank #3 (Hold). Alkermes ALKS is a better-ranked biotech stock, sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
The Zacks Consensus Estimate for Alkermes’ 2022 loss per share has narrowed from 13 cents to 3 cents in the past 60 days. Shares of ALKS have risen 14.4% year to date.
Earnings of Alkermes beat estimates in each of the last four quarters, the average being 350.48%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
To read this article on Zacks.com click here.
Zacks Investment Research
