Plus Therapeutics Announces First Patient Dosed in ReSPECT-GBM Phase 2b Trial of Rhenium (186Re) Obisbemeda for Treatment of Recurrent Glioblastoma


Figure


AUSTIN, Texas, Jan. 18, 2023 (GLOBE NEWSWIRE) -- Plus Therapeutics, Inc. (Nasdaq: PSTV) (the “Company”), a clinical-stage pharmaceutical company developing targeted radiotherapeutics with advanced platform technologies for central nervous system cancers, today announced that the first patient has been dosed in the ReSPECT-GBM Phase 2b dose expansion clinical trial evaluating rhenium (186Re) obisbemeda for the treatment of recurrent glioblastoma (GBM).
This Phase 2b multi-center trial is designed to evaluate the safety, tolerability, distribution and efficacy of rhenium (186Re) obisbemeda infused directly into the tumor via convection-enhanced delivery catheters in patients with recurrent GBM progressing after conventional treatment.
“In the Phase 1/2a dose escalation trial, we showed that a rhenium (186Re) obisbemeda radiation dose of 22.3 mCi in an infused volume of 8.8 mL can be safely administered and that there is a statistically significant correlation between overall survival and both absorbed radiation dose to the tumor and percent tumor volume in the treated volume,” said Andrew J. Brenner, M.D., Ph.D., Professor of Medicine, Neurology, and Neurosurgery, Kolitz/Zachry Endowed Chair Neuro-Oncology Research, and Co-Leader of the Experimental and Developmental Therapeutics Program at The University of Texas Health Science Center at San Antonio and principal investigator of the ReSPECT-GBM clinical trial. “The strength of this correlation is unusually positive for a Phase 1/2a trial and we are optimistic that these safety and efficacy signals will be confirmed in the ongoing Phase 2b trial.”
The Phase 2b trial is expected to enroll up to 31 additional patients with small- to medium-sized tumors (20 mL or less) in approximately 24 months. The trial is supported by an award from the National Cancer Institute (NCI), part of the U.S. National Institutes of Health (NIH).
“We have successfully completed two of our key near-term clinical development goals, specifically to manufacture cGMP rhenium (186Re) obisbemeda and move our glioblastoma program into Phase 2b,” said Norman LaFrance, M.D., Chief Medical Officer and Senior Vice President at Plus Therapeutics. “The treatment of our first patient went well with excellent targeted tumor delivery of high dose rhenium (186Re) obisbemeda, similar to that seen at this same dose in our Phase 1/2a trial. Moving forward this year, our focus will be to expand the trial sites and ramp up enrollment to accelerate clinical development of this novel treatment option.”
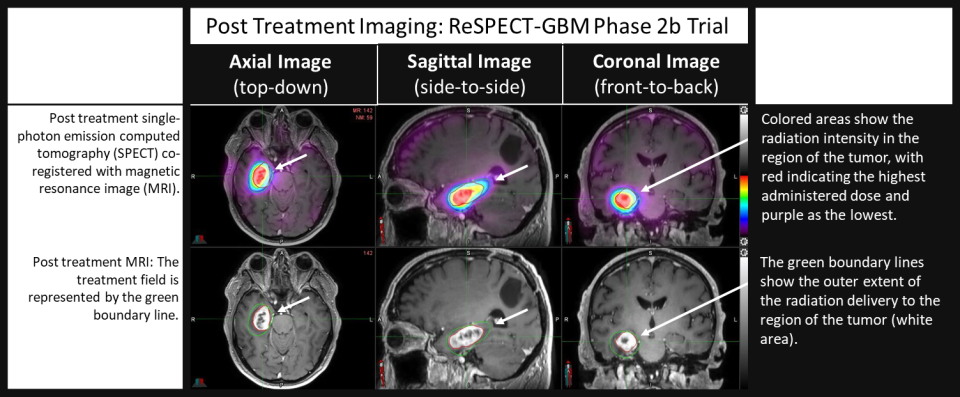
Figure: Initial treatment imaging from first patient treated in the ReSPECT-GBM Phase 2b trial.
As disclosed at the Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology in November 2022, results from the Phase 1 ReSPECT-GBM clinical trial of rhenium (186Re) obisbemeda in 24 patients with recurrent GBM demonstrated that a statistically significant improvement in overall survival correlated with both the average absorbed dose of radiation to the tumor and the percent volume of tumor treated. The treatment was safe and well tolerated without dose limiting toxicities.
The U.S. FDA granted both Orphan Drug designation and Fast Track designation to rhenium (186Re) obisbemeda for the treatment of GBM. More information about the ReSPECT-GBM trial may be found at ReSPECT-Trials.com and ClinicalTrials.gov (NCT01906385).
About Recurrent Glioblastoma (GBM)
GBM affects approximately 14,490 patients annually in the U.S. and is the most common and lethal form of brain cancer. The average life expectancy with GBM is less than 24 months, with a one-year survival rate of 40.8% and a five-year survival rate of only 6.9%. There is no clear standard of care for recurrent GBM and even the few currently approved treatments provide only marginal survival benefit and are associated with significant side effects, which limit dosing and prolonged use. Approximately 90% of patients experience GBM tumor recurrence at or near the original tumor location, yet there are no FDA-approved treatments in the recurrent or progressive setting that can significantly extend a patient’s life.
About Rhenium (186Re) obisbemeda
Rhenium (186Re) obisbemeda is a novel injectable radiotherapy specifically formulated to deliver highly targeted high dose radiation in CNS tumors in a safe, effective and convenient manner to optimize patient outcomes. Rhenium (186Re) obisbemeda has the potential to reduce risks and improve outcomes for CNS cancer patients, versus currently approved therapies, with a more targeted and potent radiation dose. Rhenium is an ideal radioisotope for CNS therapeutic applications due to its short half-life, beta energy for destroying cancerous tissue and gamma energy for live imaging.
About Plus Therapeutics
Plus Therapeutics, Inc. is a clinical-stage pharmaceutical company developing targeted radiotherapeutics for difficult-to-treat cancers of the central nervous system with the potential to enhance clinical outcomes for patients. Combining image-guided local beta radiation and targeted drug delivery approaches, the Company is advancing a pipeline of product candidates with lead programs in recurrent glioblastoma (GBM) and leptomeningeal metastases (LM). The Company has built a robust supply chain through strategic partnerships that enable the development, manufacturing and future potential commercialization of its products. Plus Therapeutics is led by an experienced and dedicated leadership team and has operations in key cancer clinical development hubs including Austin and San Antonio, Texas. For more information, visit https://plustherapeutics.com/.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains statements that may be deemed “forward-looking statements” within the meaning of U.S. securities laws. All statements in this press release other than statements of historical fact are forward-looking statements. These forward-looking statements may be identified by future verbs, as well as terms such as “designed to,” “will,” “can,” “potential,” “focus,” “preparing,” “next steps,” “possibly,” and similar expressions or the negatives thereof. Such statements are based upon certain assumptions and assessments made by management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate. These statements include, without limitation, statements regarding the following: the potential promise of 186Re including the ability of 186Re to safely and effectively deliver radiation directly to the tumor at high doses; expectations as to the Company’s future performance including the next steps in developing the Company’s current assets; the Company’s clinical trials including statements regarding the timing and characteristics of the ReSPECT-GBM and ReSPECT-LM clinical trials; possible negative effects of 186Re; the continued evaluation of 186Re including through evaluations in additional patient cohorts; and the intended functions of the Company’s platform and expected benefits from such functions.
The forward-looking statements included in this press release are subject to a number of risks and uncertainties that may cause actual results to differ materially from those discussed in such forward-looking statements. These risks and uncertainties include, but are not limited to: the Company’s actual results may differ, including materially, from those anticipated in these forward-looking statements as a result of various factors, including, but not limited to, the following: the early stage of the Company’s product candidates and therapies, the results of the Company’s research and development activities, including uncertainties relating to the clinical trials of its product candidates and therapies; the Company’s liquidity and capital resources and its ability to raise additional cash, the outcome of the Company’s partnering/licensing efforts, risks associated with laws or regulatory requirements applicable to it, market conditions, product performance, litigation or potential litigation, and competition within the cancer diagnostics and therapeutics field, among others; and additional risks described under the heading “Risk Factors” in the Company’s Securities and Exchange Commission filings, including in the Company’s annual and quarterly reports. There may be events in the future that the Company is unable to predict, or over which it has no control, and its business, financial condition, results of operations and prospects may change in the future. The Company assumes no responsibility to update or revise any forward-looking statements to reflect events, trends or circumstances after the date they are made unless the Company has an obligation under U.S. federal securities laws to do so.
Investor Contact
Peter Vozzo
ICR Westwicke
(443) 377-4767
Peter.Vozzo@westwicke.com
Media Contact
Terri Clevenger
ICR Westwicke
(203) 856-4326
Terri.Clevenger@westwicke.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f7cd08bc-103c-459d-ad98-4d319efa0b25

